FDA grants traditional approval to Eisai/Biogen Alzheimer’s drug Leqembi
Photo of Leqembi product packaging. EISAI/Handout
July 12 (ZFJ) — The Food and Drug Administration granted traditional approval to Eisai and Biogen’s Leqembi (lecanemab-irmb) 100 mg/mL intravenous injection on July 6. It is the first Alzheimer’s drug to clear this regulatory hurdle and aims to slow the disease’s progression.
“Today’s action is the first verification that a drug targeting the underlying disease process of Alzheimer’s disease has shown clinical benefit in this devastating disease,” said Teresa Buracchio, acting director of the Office of Neuroscience in FDA’s Center for Drug Evaluation and Research.
Leqembi is a monoclonal antibody treatment that targets aggregated soluble and insoluble forms of amyloid beta (Aβ) protein. Monoclonal antibodies are lab-made proteins that can bind to certain targets in the body.
Alzheimer’s disease is an irreversible brain disorder that gradually destroys a patient’s memory, thinking skills, and ability to perform simple tasks. It affects over 6.5 million Americans.
The cause of Alzheimer’s remains unknown, but the disease is characterized by the formation of amyloid beta plaques and neurofibrillary tangles, which results in the loss of neurons, which transmit information in the nervous system. Current research suggests that removing these plaques can slow the disease’s progression.
The FDA first granted Leqembi accelerated approval on January 6, 2023. According to the medicine agency, this approval allows regulators to “approve drugs for serious conditions where there is an unmet medical need, based on clinical data demonstrating the drug’s effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit to patients.”
As a condition of accelerated approval, the FDA required the pharmaceutical companies to conduct a phase 3 study to confirm Leqembi’s efficacy in slowing Alzheimer’s.
The study, Study 301 (Clarity AD), involved 1,795 patients and was double-blind and placebo-controlled. Patients were randomized in a 1:1 ratio to receive either a placebo or Leqembi. The study’s results were published in The New England Journal of Medicine.
The study found that Leqembi resulted in a statistically significant reduction of progression in the primary endpoint, the Clinical Dementia Rating-Sum of Boxes score, from baseline to 18 months. The CDR-SB score assesses cognition and function based on interviews with the patients. Higher scores reflect greater impairment.
The study also found statistically significant differences on all secondary endpoints, including the amount of amyloid beta plaques remaining, Alzheimer’s Disease Assessment Scale Cognitive Subscale 14 (ADAS-cog14), and Alzheimer’s Disease Cooperative Study-Activities of Daily Living Scale for Mild Cognitive Impairment (ADCS-MCI-ADL).
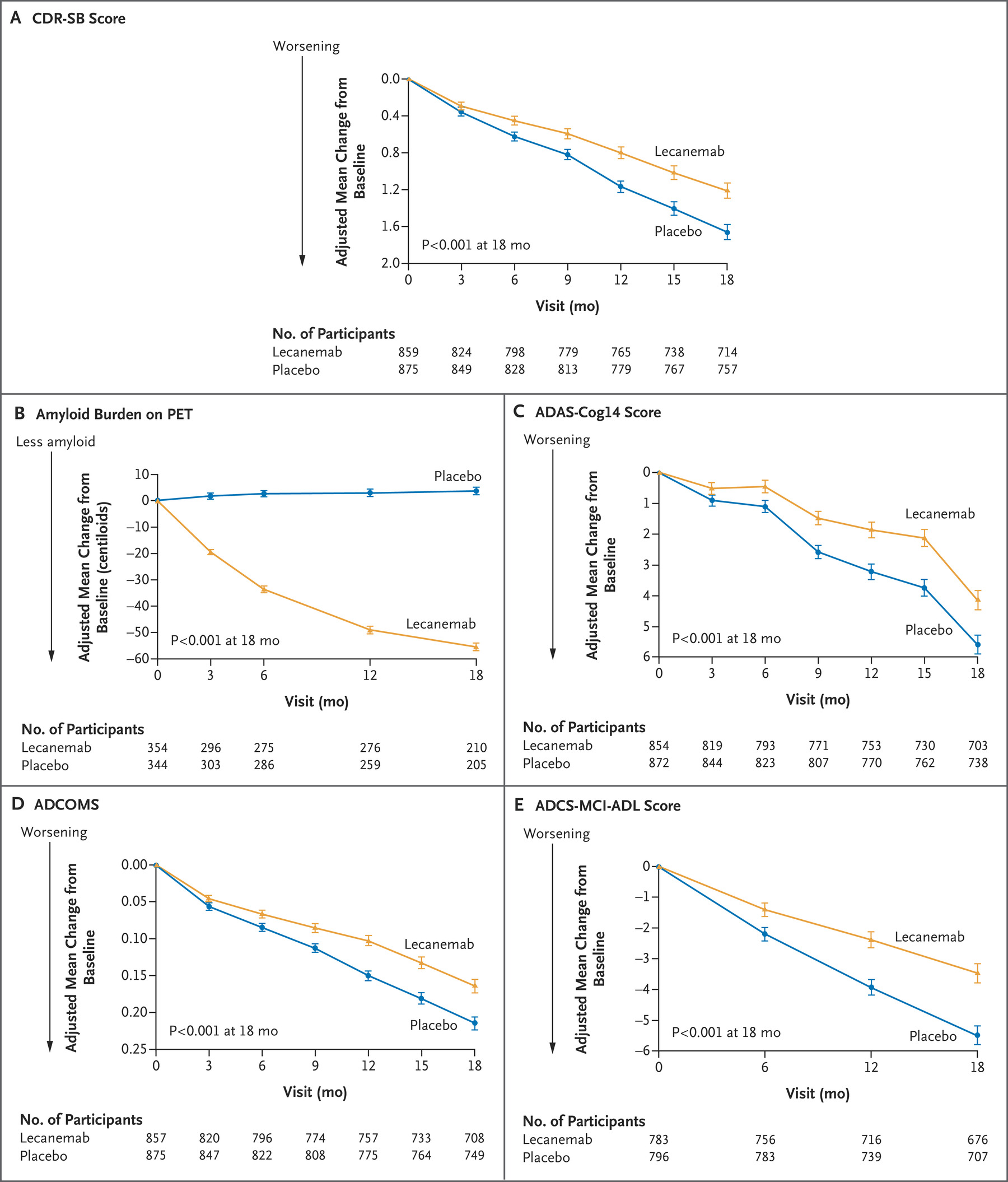 Graphs showing primary and secondary endpoint results of the phase 3 study Study 301 (Clarity AD) concerning Leqembi’s effectiveness. THE NEW ENGLAND JOURNAL OF MEDICINE/Graphic
Graphs showing primary and secondary endpoint results of the phase 3 study Study 301 (Clarity AD) concerning Leqembi’s effectiveness. THE NEW ENGLAND JOURNAL OF MEDICINE/Graphic
Leqembi is meant for patients with mild cognitive impairment or mild dementia stage of Alzheimer’s. This population was studied in the clinical trial. There is no data on its safety or efficacy at earlier or later stages of the disease.
The most common adverse events from Leqembi use in the study were infusion-related reactions (26.4% who received lecanemab affected), ARIA (17.3%), ARIA-E (12.6%), headache (11.1%), and falls (10.4%). ARIA stands for amyloid-related imaging abnormalities and often presents with temporary brain swelling and small spots of bleeding. It can infrequently present with life-threatening brain edema (excess fluid accumulation) or fatal intracerebral hemorrhages.
Patients who are homozygous for (have two copies of) the ApoE ε4 allele (form of a gene) are more likely to have ARIA.
With these results, the eight members of the Peripheral and Central Nervous System Drugs Advisory Committee, an FDA panel of independent experts, voted in favor of approving the drug on June 9, 2023. The committee currently has three vacancies.
“Today marks a breakthrough in the treatment of Alzheimer’s disease, and we are proud to be at the forefront of ushering in a new era of advances for a disease that was previously considered untreatable,” said Biogen President and CEO Christopher A. Viehbacher.
The approval also means that Medicare will now cover Leqembi.
Patients enrolled in Medicare who are diagnosed with mild cognitive impairment or mild dementia, have documented evidence of amyloid-beta plaques, and have a physician participating in a qualifying registry are eligible for coverage, said the Centers for Medicare and Medicaid Services.
Leqembi will cost $26,500 per year. Eisai estimates out-of-pocket costs for patients to range from $0.00 to $14.50 per day, depending on one’s insurance.
Leqembi’s approval was granted to Eisai, the lead of the drug’s development and regulatory submissions. Eisai and Biogen both co-commercialize and co-promote it.
Leqembi’s approval in Japan, the European Union, China, Canada, Great Britain, and South Korea is pending.
One anti-amyloid antibody, Biogen’s Aduhelm (aducanumab), previously received accelerated approval from the FDA on June 7, 2021.
This decision was controversial because the independent nervous system drugs advisory committee overwhelmingly voted against doing so. Two phase 3 clinical trials were conducted for Aduhelm, one of which did demonstrate slower Alzheimer’s progression, and one of which did not support the drug’s effectiveness, although both studies showed it reduced amyloid plaque levels in patients.
Three members of the advisory committee resigned in response to Aduhelm’s approval.
References
- U.S. Food and Drug Administration - FDA Converts Novel Alzheimer’s Disease Treatment to Traditional Approval - https://www.fda.gov/news-events/press-announcements/fda-converts-novel-alzheimers-disease-treatment-traditional-approval (ARCHIVE)
- U.S. Food and Drug Administration - FDA’s Decision to Approve New Treatment for Alzheimer’s Disease - https://www.fda.gov/drugs/news-events-human-drugs/fdas-decision-approve-new-treatment-alzheimers-disease (ARCHIVE)
- Eisai - FDA Grants Traditional Approval for LEQEMBI® (lecanemab-irmb) for the Treatment of Alzheimer’s Disease - https://www.eisai.com/news/2023/news202349.html (ARCHIVE)
- The New England Journal of Medicine - Lecanemab in Early Alzheimer’s Disease - https://www.nejm.org/doi/full/10.1056/NEJMoa2212948 (ARCHIVE)
- Centers for Medicare and Medicaid Services - Statement: Broader Medicare Coverage of Leqembi Available Following FDA Traditional Approval - https://www.cms.gov/newsroom/press-releases/statement-broader-medicare-coverage-leqembi-available-following-fda-traditional-approval (ARCHIVE)
- National Cancer Institute - monoclonal antibody - https://www.cancer.gov/publications/dictionaries/cancer-terms/def/monoclonal-antibody (ARCHIVE)
- ClinicalTrials.gov - ClinicalTrials.gov Glossary Terms - https://clinicaltrials.gov/study-basics/glossary (ARCHIVE)
- ClinicalTrials.gov - A Study to Confirm Safety and Efficacy of Lecanemab in Participants With Early Alzheimer’s Disease (Clarity AD) - https://clinicaltrials.gov/study/NCT03887455 (ARCHIVE)